Case Studies: Examine EXOGEN Outcomes
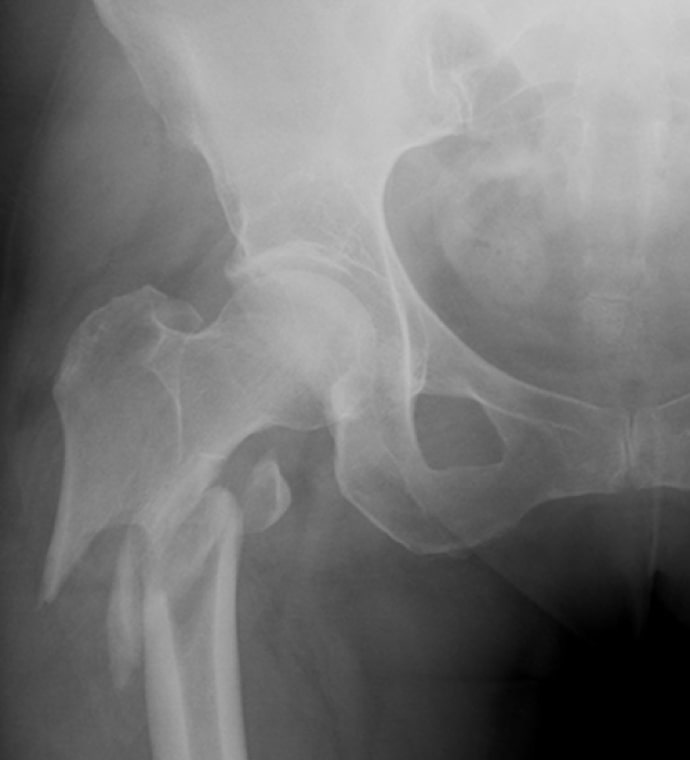
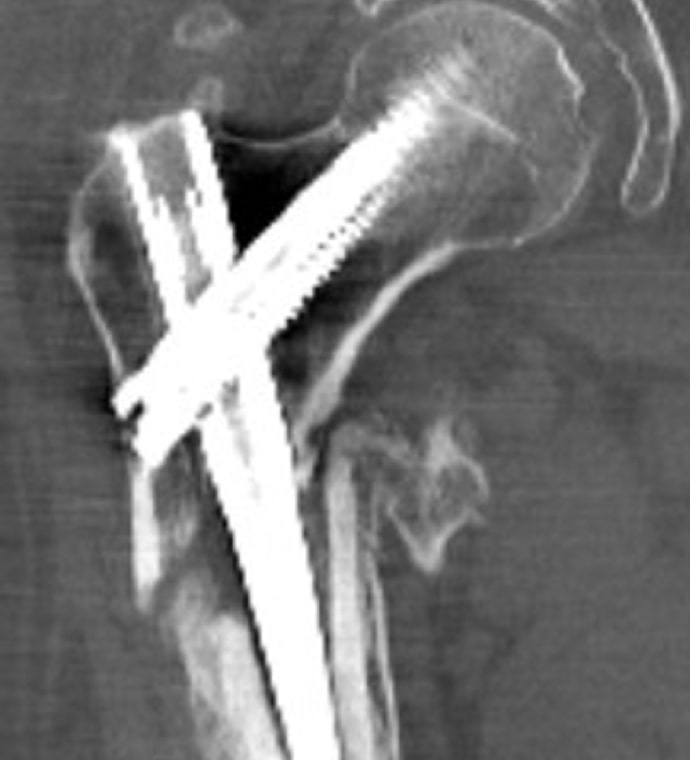
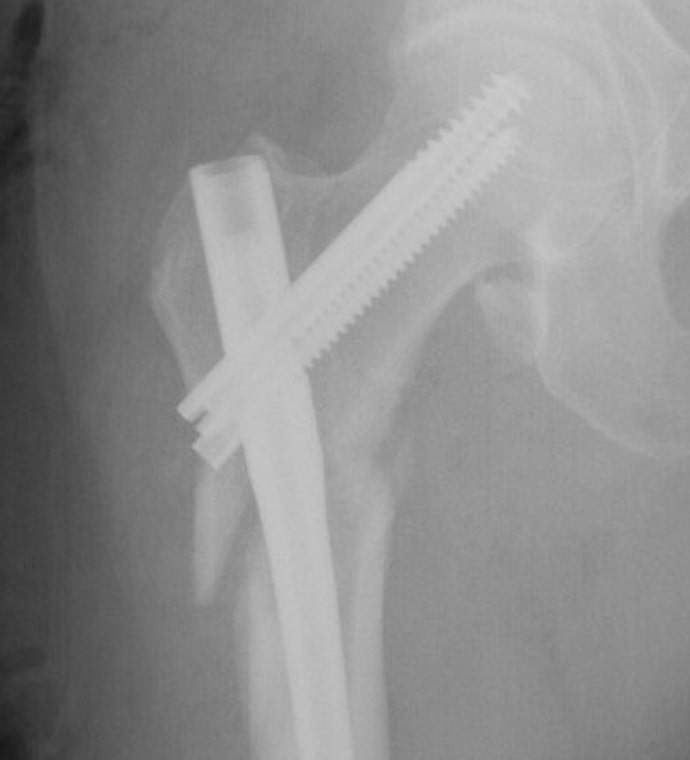
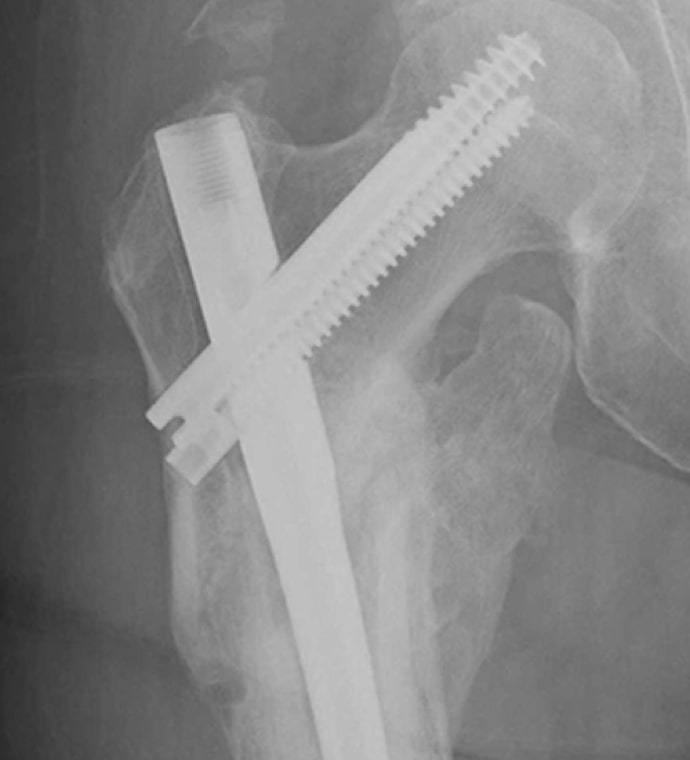
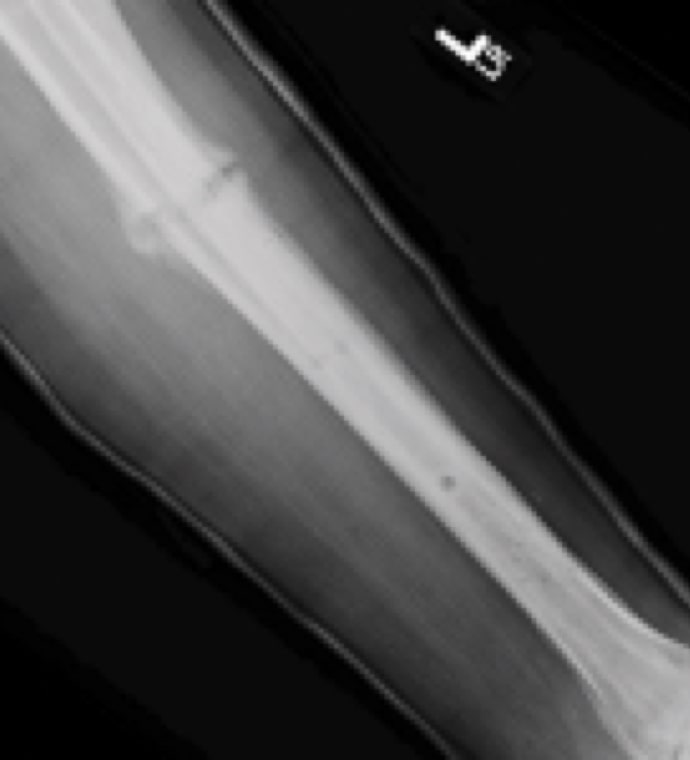
Case Study Background
Patient Story
- 47-year-old female
- Tripped on uneven pavement
- Right reverse obliquity subtrochanteric femur fracture
Source of Patient Case
Michael Prayson, MD
Professor and Vice Chair
Wright State University
Dayton, OH
Risk Factors
- Smoking history
- Obesity
- Fracture pattern
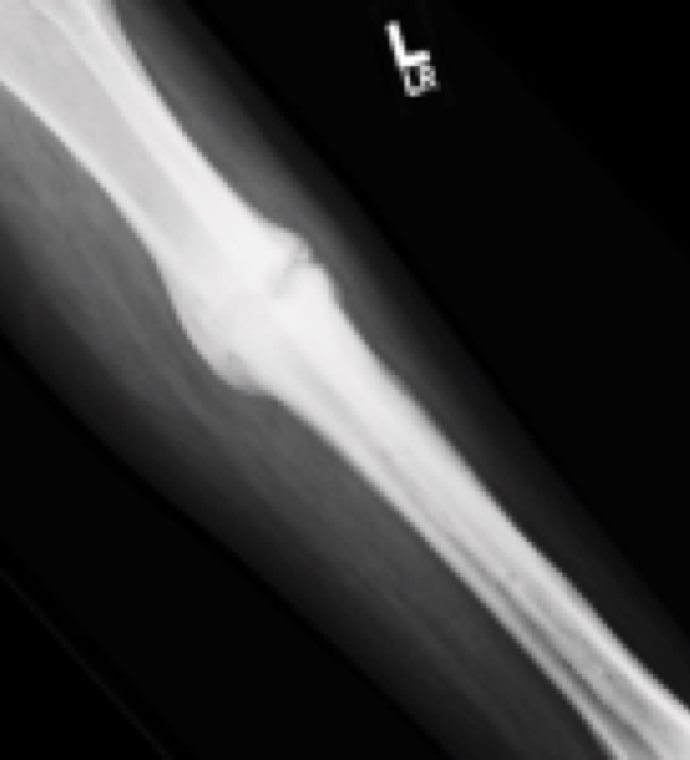
Case Study Background
Patient Story
- 53-year-old female school principal
- History of nonunion for prior midfoot deformity correction
Source of Patient Case
Robert Anderson, MD
OrthoCarolina Foot & Ankle Institute
Charlotte, NC
Initial History
- Tibia fracture at external fixation pin site
- Closed reduction with long-leg cast
- Non-weight bearing
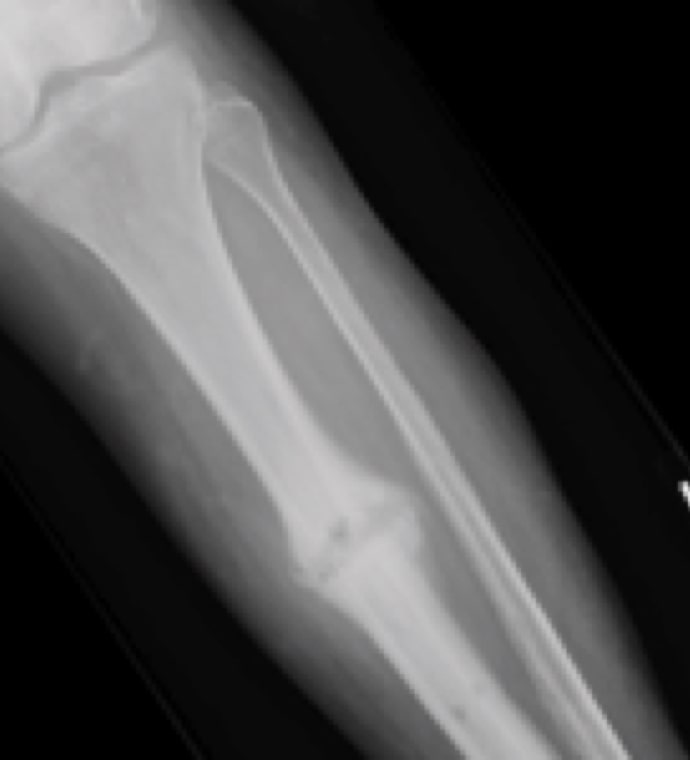
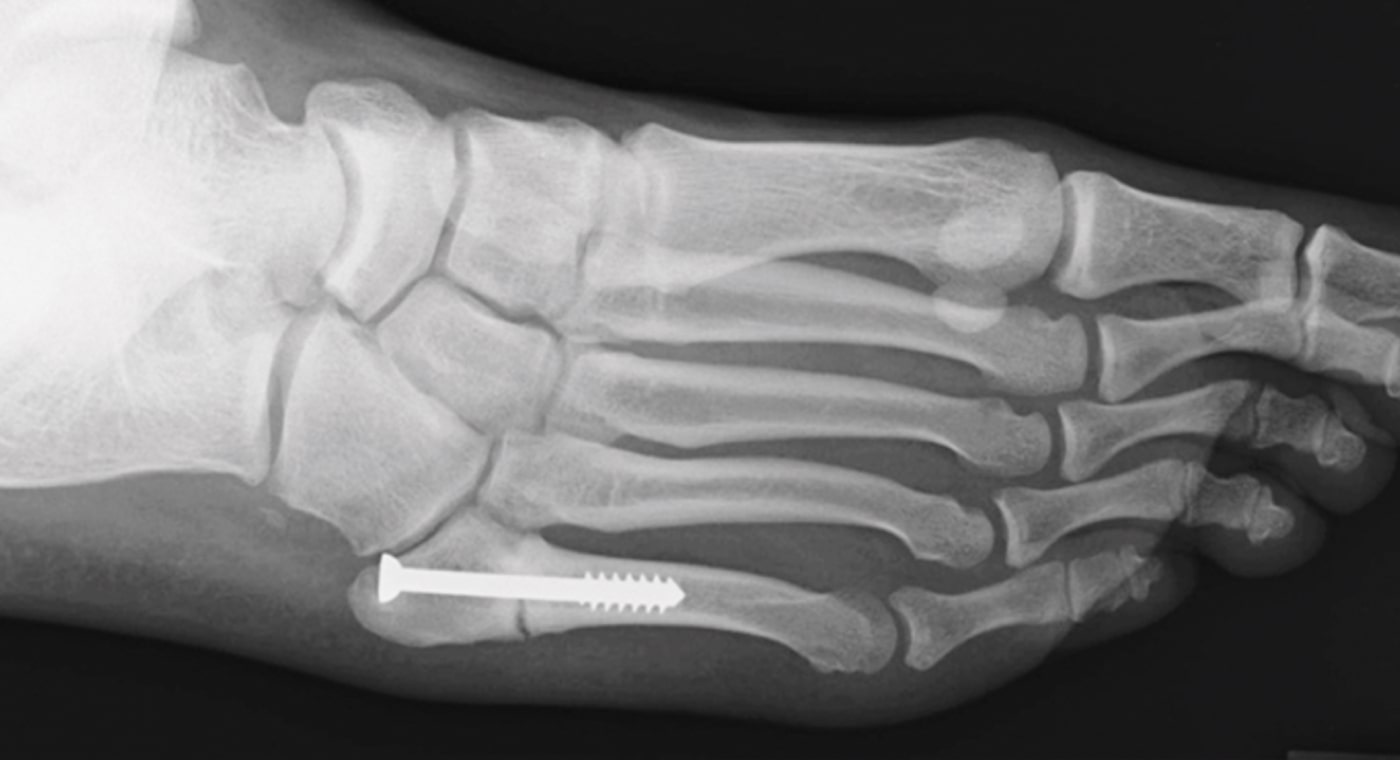
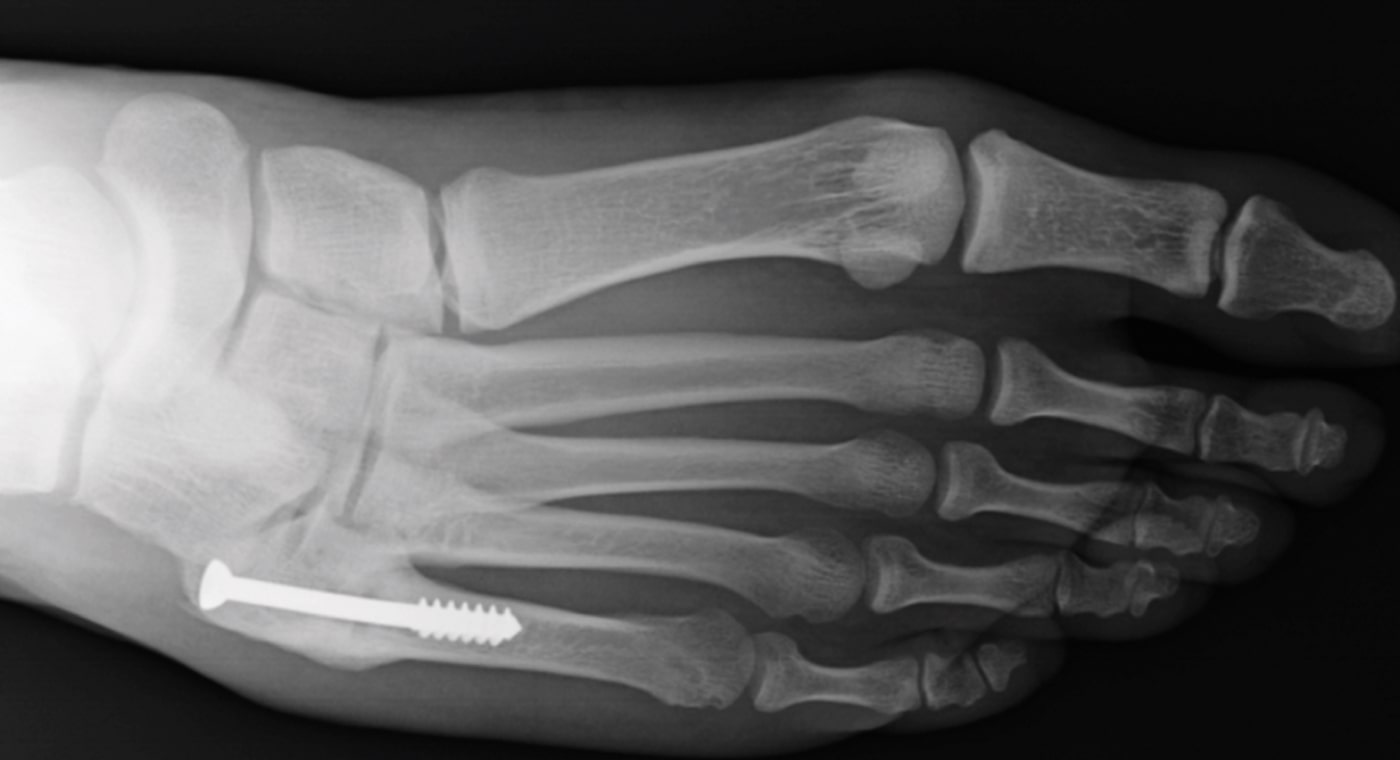
Case Study Background
Patient Story
- 20-year-old male college football player
- History of Jones fracture
- Two prior surgeries
- No comorbidities
Source of Patient Case
Robert Anderson, MD
OrthoCarolina Foot & Ankle Institute
Charlotte, NC
Initial History
- Motor vehicle accident
- Refractured metatarsal on right foot
- Treated conservatively with cast and boot
Summary of Indications for Use
The EXOGEN Ultrasound Bone Healing System is indicated for the non-invasive treatment of established nonunions* excluding skull and vertebra. The EXOGEN device has also been reported as effective as an adjunctive non-invasive treatment of established nonunions in patients:
- With internal or external fracture fixation hardware present. EXOGEN cannot penetrate metal and therefore should not be applied directly over hardware.
- Undergoing treatment for infection at the fracture site. EXOGEN is not intended to treat the infection.
- Believed to have diminished bone quality. EXOGEN is not intended to treat diminished bone quality.
In addition, EXOGEN is indicated for accelerating the time to a healed fracture for fresh, closed, posteriorly displaced distal radius fractures and fresh, closed or Grade I open tibial diaphysis fractures in skeletally mature individuals when these fractures are orthopedically managed by closed reduction and cast immobilization.
There are no known contraindications for the EXOGEN device. Safety and effectiveness have not been established for individuals lacking skeletal maturity, pregnant or nursing women, patients with cardiac pacemakers, on fractures due to bone cancer, or on patients with poor blood circulation or clotting problems. Some patients may be sensitive to the ultrasound gel.
*A nonunion is considered to be established when the fracture site shows no visibly progressive signs of healing.
Full prescribing information can be found in product labelling, at EXOGEN.com, or by calling Customer Service at 1-800-836-4080.