Expect More for Your Patients
Many patients have difficult-to-heal fractures, or fractures they want to heal more quickly. EXOGEN offers a non-surgical option that helps to heal nonunion fractures and accelerates indicated acute fractures, so your patients can resume active lifestyles.*1-3
EXOGEN is a low-intensity pulsed ultrasound (LIPUS) bone stimulator with over 25 years of proven noninvasive bone healing.72 Prescribed by more than 10,000 physicians annually to help over a million patients worldwide, EXOGEN has an 86% nonunion heal rate and accelerates the healing of indicated acute fractures by 38%.1-4,12
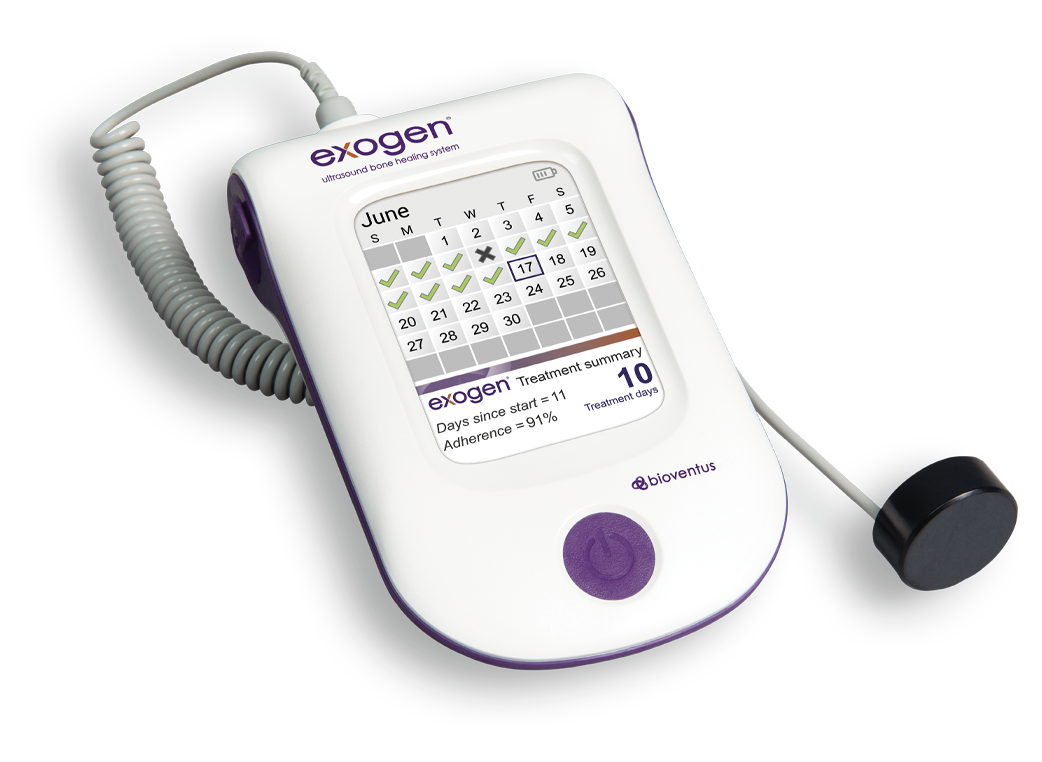
EXOGEN makes treatment compliance tracking convenient for your patients and verifiable for you, with a built-in treatment tracking calendar.
Explore EXOGEN's Proven Results
The EXOGEN Ultrasound Bone Healing System is the first FDA-approved device with 38% accelerated healing of indicated acute fractures.1,2
Outcomes with EXOGEN:
Accelerated healing in cortical and cancellous bone:
- 58 days and 37 days faster, respectively1,2
- Fracture healing promoted 41% to 51% faster in patients who smoke9
Accelerated healing of indicated fresh tibia fractures in younger and older patients1,52
- 42 days and 84 days faster, respectively1,52
Discover the noninvasive choice for resolution of nonunions. In clinical studies, low-intensity pulsed ultrasound waves helped heal nonunion fractures.
EXOGEN is effective:
- In chronic nonunion fractures that failed to heal at least one year prior to treatment – 86% heal rate35
- In atrophic, hypertrophic, or infected nonunions29,41,53
- With challenging, established nonunions – 86% heal rate3
- With high-energy nonunion fractures – 89% heal rate7
- For use on nonunions with an interfragment gap up to 10 mm and stable osteosynthesis19
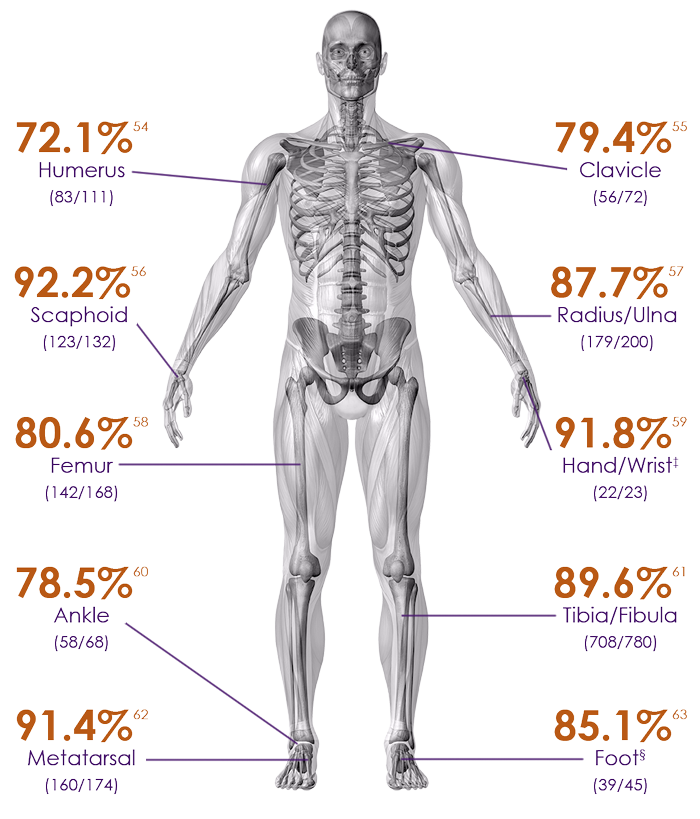
Heal rate of common fractures at risk for nonunions8
In many cases, EXOGEN may prevent the need for further surgery.
Humerus – 72.1% heal rate54
Clavicle – 79.4% heal rate55
Scaphoid – 92.2% heal rate56
Radius/Ulna – 87.7% heal rate57
Femur – 80.6% heal rate58
Hand/wrist – 91.8% heal rate‡59
Ankle – 78.5% heal rate60
Tibia/Fibula – 89.6% heal rate61
Metatarsal – 91.4% heal rate62
Foot – 85.1% heal rate§63
Heal rates based on fractures ages between 91-365 days.54-63
‡Includes metacarpal, carpal and hamate
§Includes talus, calcaneus, tarsal navicular, cuboid and cuneiform
**These studies, which reflect the body of evidence of the bone stimulator EXOGEN, include evaluations of applications outside the approved label. Assignment of evidence levels was based on the updated level of evidence rating system in the Oxford Level of Evidence Criteria. Oxford Centre for Evidence-Based Medicine; www.cebm.ox.ac.uk/resources/levels-of-evidence/ocebm-levels-of-evidence
*Summary of Indications for Use
The EXOGEN Ultrasound Bone Healing System is indicated for the non-invasive treatment of established nonunions† excluding skull and vertebra. The EXOGEN device has also been reported as effective as an adjunctive non-invasive treatment of established nonunions in patients:
- With internal or external fracture fixation hardware present. EXOGEN cannot penetrate metal and therefore should not be applied directly over hardware.
- Undergoing treatment for infection at the fracture site. EXOGEN is not intended to treat the infection.
- Believed to have diminished bone quality. EXOGEN is not intended to treat diminished bone quality.
In addition, EXOGEN is indicated for accelerating the time to a healed fracture for fresh, closed, posteriorly displaced distal radius fractures and fresh, closed or Grade I open tibial diaphysis fractures in skeletally mature individuals when these fractures are orthopedically managed by closed reduction and cast immobilization.
There are no known contraindications for the EXOGEN device. Safety and effectiveness have not been established for individuals lacking skeletal maturity, pregnant or nursing women, patients with cardiac pacemakers, on fractures due to bone cancer, or on patients with poor blood circulation or clotting problems. Some patients may be sensitive to the ultrasound gel.
†A nonunion is considered to be established when the fracture site shows no visibly progressive signs of healing.
Full prescribing information can be found in product labelling, at EXOGEN.com, or by calling Customer Service at 1-800-836-4080.
