Patient Case Studies
Case Studies: Examine EXOGEN Outcomes
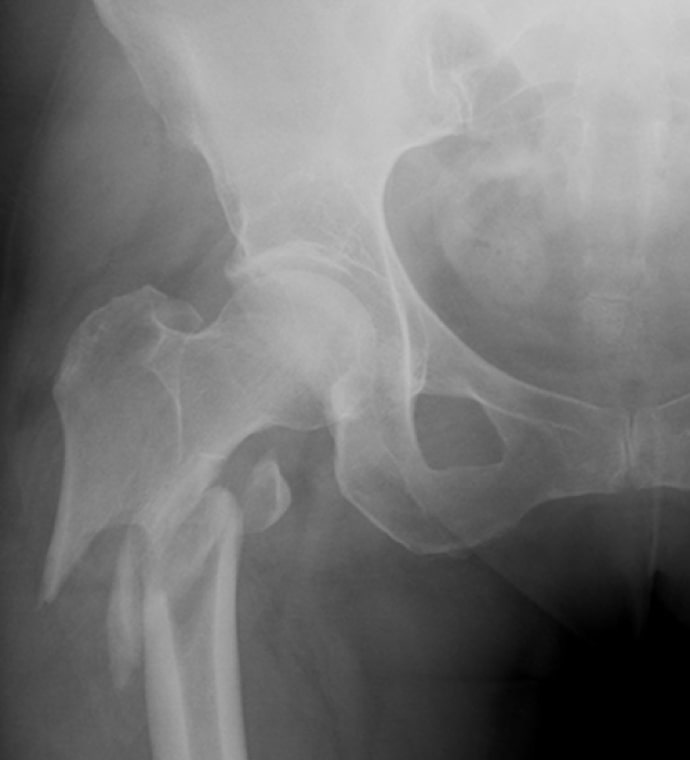
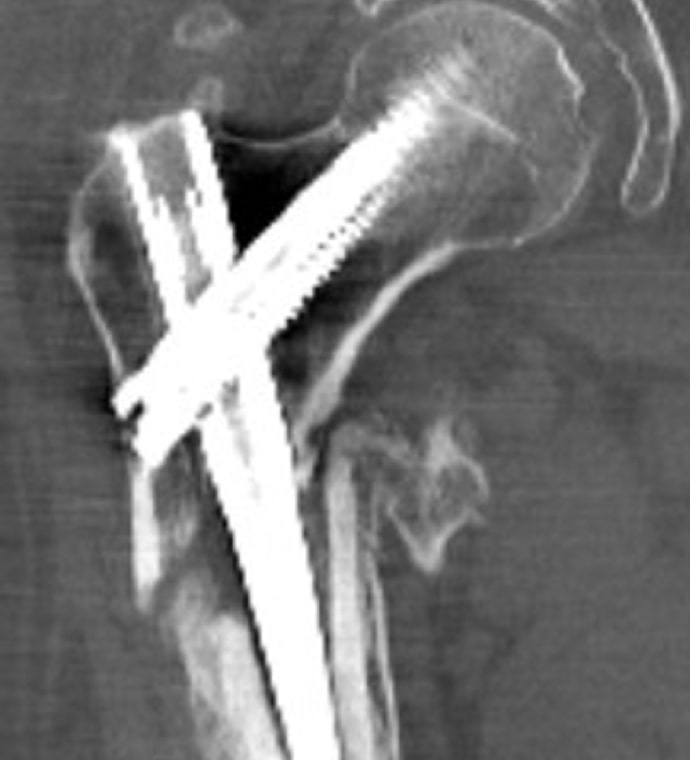
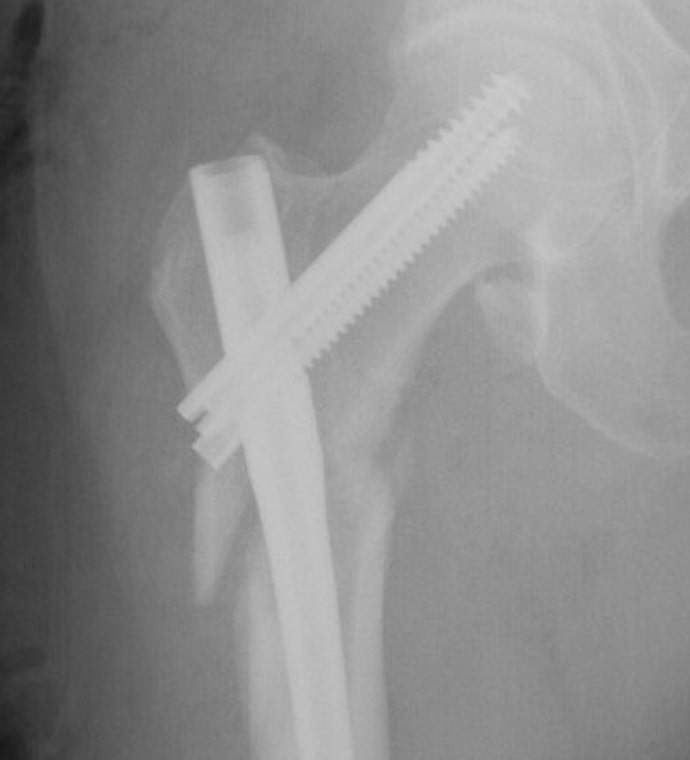
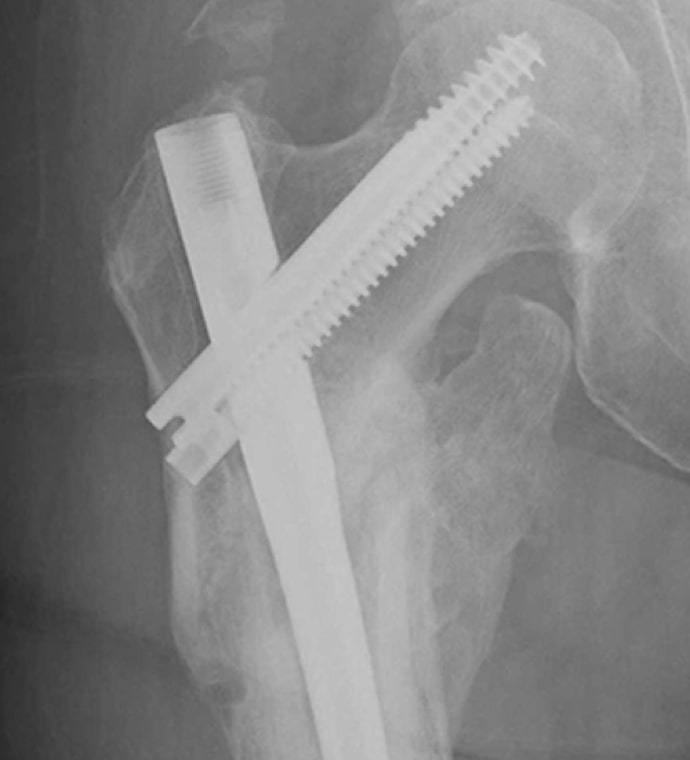
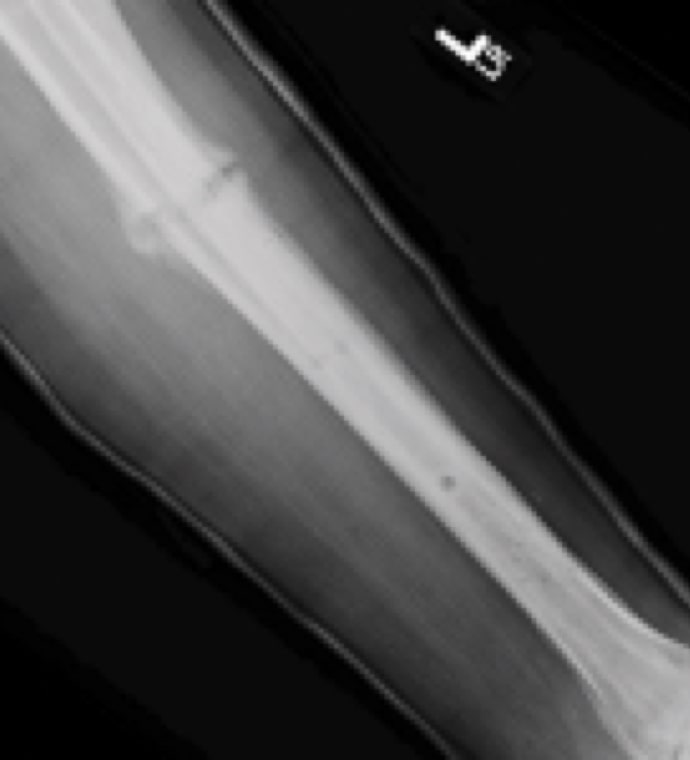
Case Study Background
Patient Story
- 47-year-old female
- Tripped on uneven pavement
- Right reverse obliquity subtrochanteric femur fracture
Source of Patient Case
Michael Prayson, MD
Professor and Vice Chair
Wright State University
Dayton, OH
Risk Factors
- Smoking history
- Obesity
- Fracture pattern
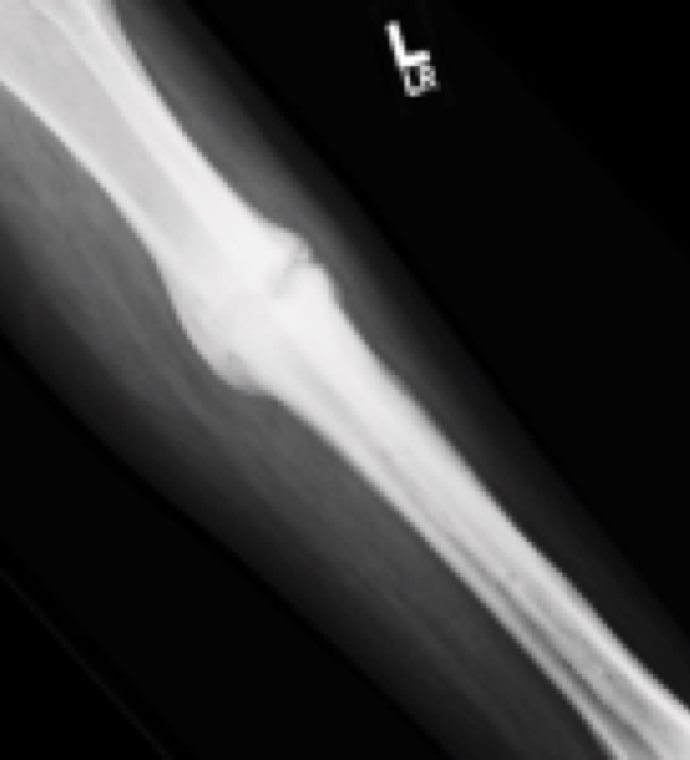
Case Study Background
Patient Story
- 53-year-old female school principal
- History of nonunion for prior midfoot deformity correction
Source of Patient Case
Robert Anderson, MD
OrthoCarolina Foot & Ankle Institute
Charlotte, NC
Initial History
- Tibia fracture at external fixation pin site
- Closed reduction with long-leg cast
- Non-weight bearing
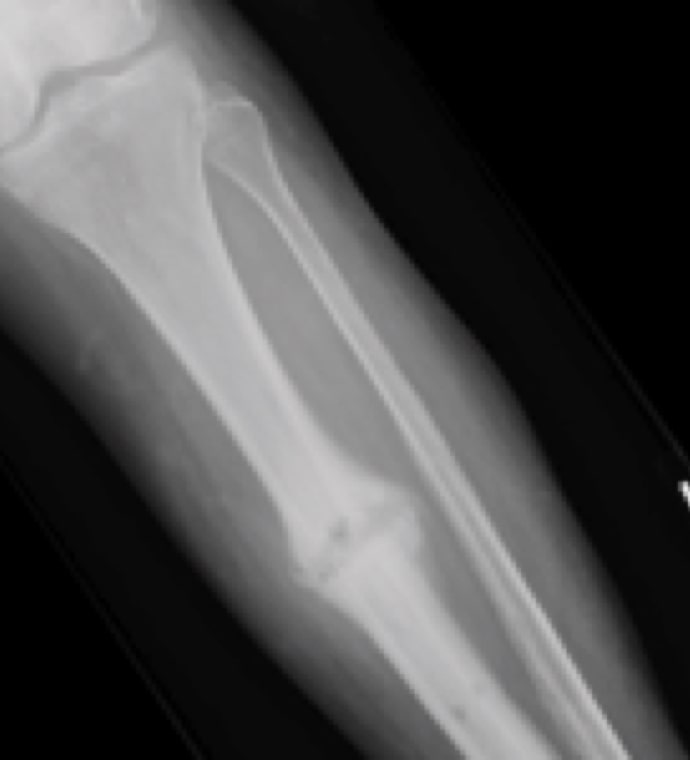
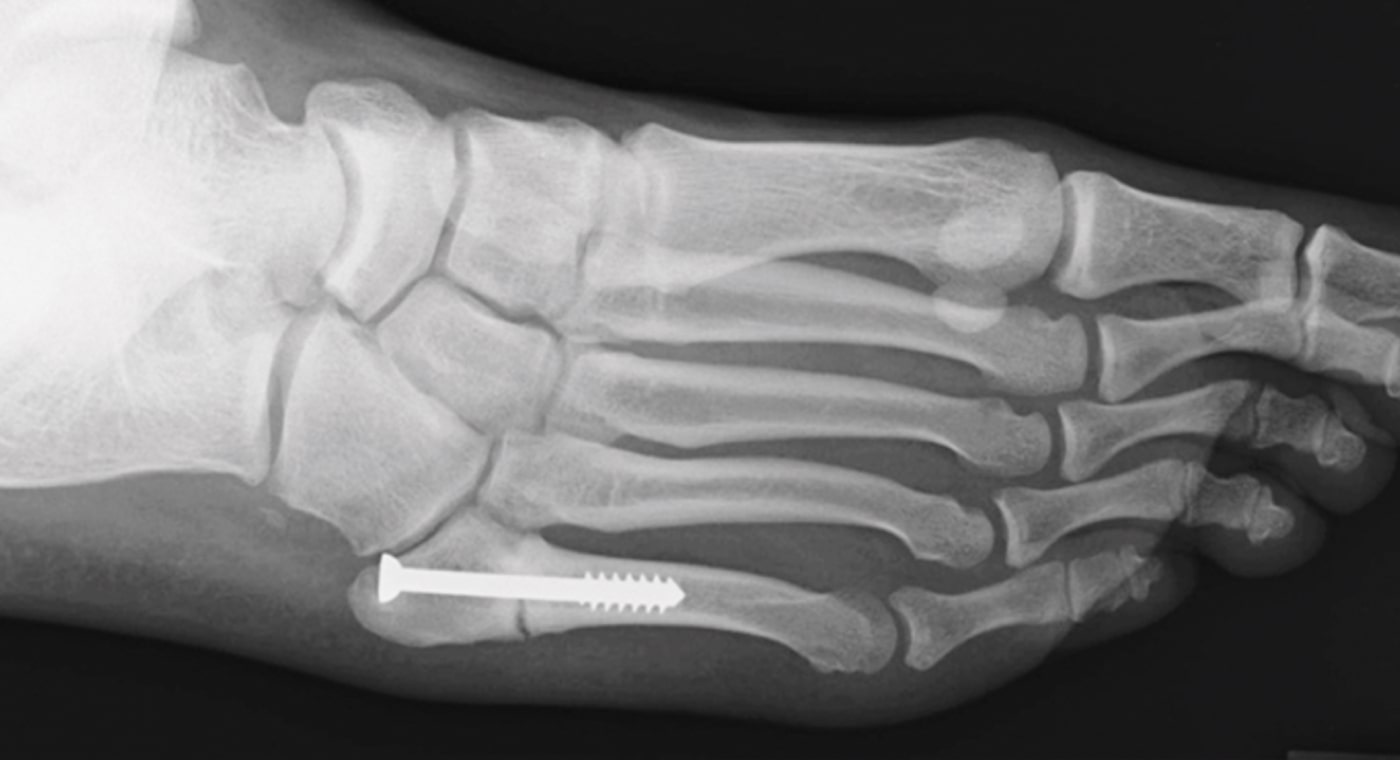
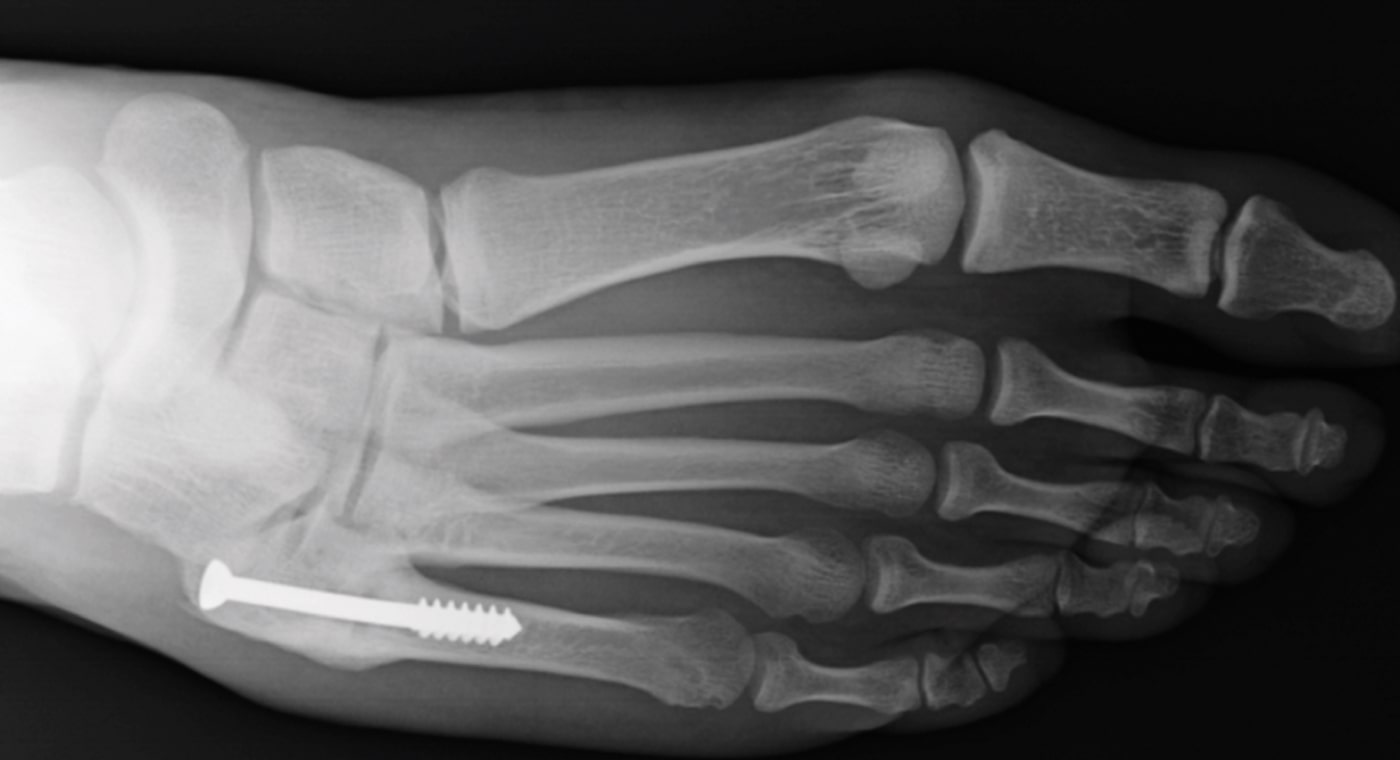
Case Study Background
Patient Story
- 20-year-old male college football player
- History of Jones fracture
- Two prior surgeries
- No comorbidities
Source of Patient Case
Robert Anderson, MD
OrthoCarolina Foot & Ankle Institute
Charlotte, NC
Initial History
- Motor vehicle accident
- Refractured metatarsal on right foot
- Treated conservatively with cast and boot
Summary of Indications for Use
The EXOGEN Ultrasound Bone Healing System is indicated for the non-invasive treatment of established nonunions* excluding skull and vertebra. The EXOGEN device has also been reported as effective as an adjunctive non-invasive treatment of established nonunions in patients:
- With internal or external fracture fixation hardware present. EXOGEN cannot penetrate metal and therefore should not be applied directly over hardware.
- Undergoing treatment for infection at the fracture site. EXOGEN is not intended to treat the infection.
- Believed to have diminished bone quality. EXOGEN is not intended to treat diminished bone quality.
In addition, EXOGEN is indicated for accelerating the time to a healed fracture for fresh, closed, posteriorly displaced distal radius fractures and fresh, closed or Grade I open tibial diaphysis fractures in skeletally mature individuals when these fractures are orthopedically managed by closed reduction and cast immobilization.
There are no known contraindications for the EXOGEN device. Safety and effectiveness have not been established for individuals lacking skeletal maturity, pregnant or nursing women, patients with cardiac pacemakers, on fractures due to bone cancer, or on patients with poor blood circulation or clotting problems. Some patients may be sensitive to the ultrasound gel.
*A nonunion is considered to be established when the fracture site shows no visibly progressive signs of healing.
Full prescribing information can be found in product labelling, at EXOGEN.com, or by calling Customer Service at 1-800-836-4080.
Physician @Home Experience
Expect Morefrom Your Patients'
@Home Experience
Easy and Convenient to Use, EXOGEN Delivers on Expectations for Bone Healing
The EXOGEN @Home Experience provides virtual placement of the EXOGEN device. Patients will receive EXOGEN delivered directly to their home by mail. Once the patient receives the device, a dedicated team member will guide them on how to use the device.
EXOGEN @Home Experience is as Easy as 1-2-3
The EXOGEN @Home Experience has a portfolio of tools available to assist with fracture treatment.
Mechanism of Action
Provides an in-depth look at how EXOGEN stimulates bone healing.
Casting Application Guide
Provides step-by-step instructions on how to build a cast for use with EXOGEN.
Weighted Applicator Strap
Provides instructions on how to assemble and use the weighted applicator strap.
Summary of Indications for Use
The EXOGEN Ultrasound Bone Healing System is indicated for the non-invasive treatment of established nonunions* excluding skull and vertebra. The EXOGEN device has also been reported as effective as an adjunctive non-invasive treatment of established nonunions in patients:
- With internal or external fracture fixation hardware present. EXOGEN cannot penetrate metal and therefore should not be applied directly over hardware.
- Undergoing treatment for infection at the fracture site. EXOGEN is not intended to treat the infection.
- Believed to have diminished bone quality. EXOGEN is not intended to treat diminished bone quality.
In addition, EXOGEN is indicated for accelerating the time to a healed fracture for fresh, closed, posteriorly displaced distal radius fractures and fresh, closed or Grade I open tibial diaphysis fractures in skeletally mature individuals when these fractures are orthopedically managed by closed reduction and cast immobilization.
There are no known contraindications for the EXOGEN device. Safety and effectiveness have not been established for individuals lacking skeletal maturity, pregnant or nursing women, patients with cardiac pacemakers, on fractures due to bone cancer, or on patients with poor blood circulation or clotting problems. Some patients may be sensitive to the ultrasound gel.
*A nonunion is considered to be established when the fracture site shows no visibly progressive signs of healing.
Full prescribing information can be found in product labelling, at EXOGEN.com, or by calling Customer Service at 1-800-836-4080.
Frequently Asked Questions
Indications for Use
EXOGEN is indicated for the noninvasive treatment of established nonunions, excluding skull and vertebra. It has been shown to accelerate the time to a healed fracture for fresh, closed, posteriorly displaced distal radius fractures and fresh, closed or Grade I open tibial diaphysis fractures in skeletally mature individuals when these fractures are orthopaedically managed by closed reduction and cast immobilization.
EXOGEN has no known contraindications.
The current EXOGEN product label includes treatment indications that have been approved for use by the FDA. FDA approval is based on reviews of extensive clinical data sponsored by Bioventus to support the effectiveness and safety of EXOGEN in these indications. “Unapproved use” refers to conditions for which a specific approved indication has not yet been granted by the FDA. For EXOGEN, many “unapproved uses” have not been adequately studied for effectiveness and safety by Bioventus to an extent that would result in FDA approval.
Fracture Treatment
Yes, the biological effect of the ultrasound beam goes around the entire fracture by shear waves.65
EXOGEN ultrasound waves can penetrate through skin and soft tissue for an effective penetration depth of ~10 inches (250 mm).65
Active, implantable devices such as cardiac pacemakers may be adversely affected by close exposure to the EXOGEN device. Patients or other people in close proximity during treatment should be evaluated by a cardiologist or physician before using the EXOGEN device.
Mark the fracture location on your patient. The EXOGEN transducer must be in direct contact with skin, so if your patient needs or currently has a cast, you’ll need to build in a transducer port or create a window. Our trained EXOGEN specialists will assist you with proper placement of the transducer port for casting and use with metals for nonunion fractures. For more information, download the EXOGEN Casting Application Techniques or contact Bioventus Customer Service at 1-800-836-4080.
EXOGEN Device & Coverage
The EXOGEN unit consists of the main operating unit and the attached cable with transducer head. Patients also receive an application strap, coupling gel, and battery charger.
Many insurers cover the EXOGEN device.13-16 For more information, contact your local EXOGEN representative or contact Bioventus Customer Service at 1-800-836-4080.
Use HCPCS code E0760 and include a description of the device: EXOGEN Ultrasound Bone Healing System by Bioventus.

Can’t find an answer to your question?
Contact us directly to speak to a representative.
Summary of Indications for Use
The EXOGEN Ultrasound Bone Healing System is indicated for the non-invasive treatment of established nonunions* excluding skull and vertebra. The EXOGEN device has also been reported as effective as an adjunctive non-invasive treatment of established nonunions in patients:
- With internal or external fracture fixation hardware present. EXOGEN cannot penetrate metal and therefore should not be applied directly over hardware.
- Undergoing treatment for infection at the fracture site. EXOGEN is not intended to treat the infection.
- Believed to have diminished bone quality. EXOGEN is not intended to treat diminished bone quality.
In addition, EXOGEN is indicated for accelerating the time to a healed fracture for fresh, closed, posteriorly displaced distal radius fractures and fresh, closed or Grade I open tibial diaphysis fractures in skeletally mature individuals when these fractures are orthopedically managed by closed reduction and cast immobilization.
There are no known contraindications for the EXOGEN device. Safety and effectiveness have not been established for individuals lacking skeletal maturity, pregnant or nursing women, patients with cardiac pacemakers, on fractures due to bone cancer, or on patients with poor blood circulation or clotting problems. Some patients may be sensitive to the ultrasound gel.
*A nonunion is considered to be established when the fracture site shows no visibly progressive signs of healing.
Full prescribing information can be found in product labelling, at EXOGEN.com, or by calling Customer Service at 1-800-836-4080.
The Science Behind EXOGEN
The Science Behind EXOGEN
The EXOGEN Bone Healing System uses FDA-approved safe, painless, low-intensity ultrasound waves to amplify your patient’s natural bone repair processes.68
Mechanism of Action



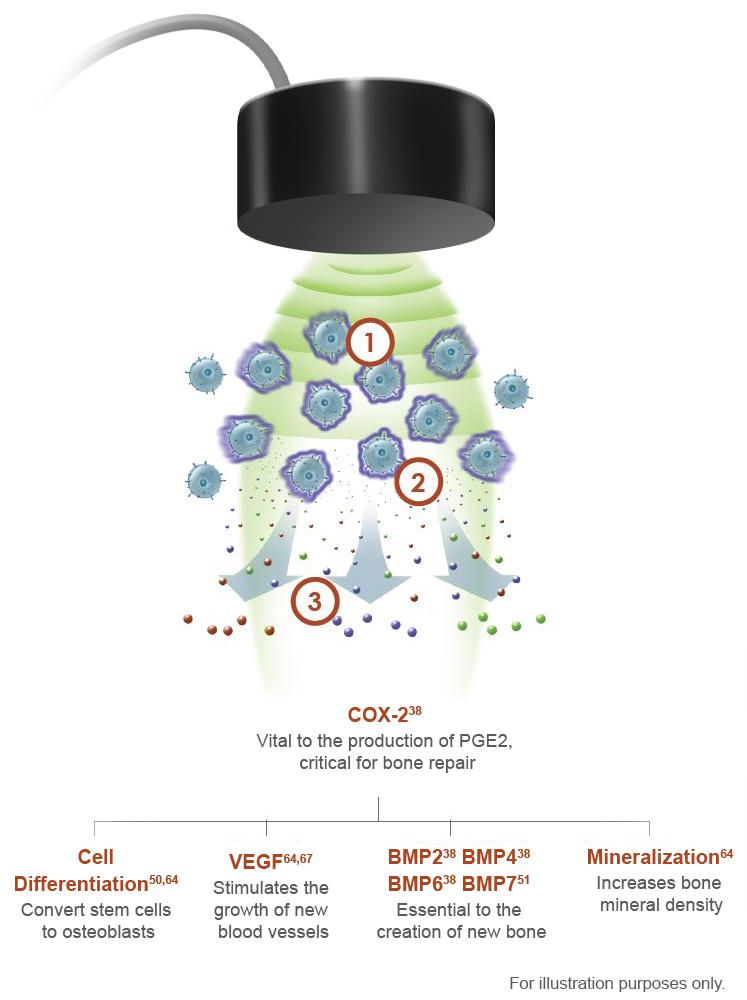
EXOGEN has Multiple Level 1 and 2 Clinical Studies*1-3,5,18-35
*These studies, which reflect the body of evidence of the bone stimulator EXOGEN, include evaluations of applications outside the approved label. Assignment of evidence levels was based on the updated level of evidence rating system in the Oxford Level of Evidence Criteria. Oxford Centre for Evidence-Based Medicine; www.cebm.ox.ac.uk/resources/levels-of-evidence/ocebm-levels-of-evidence
*The clinical relevance of in vivo findings is unknown
*The clinical relevance of in vivo findings is unknown
Summary of Indications for Use
The EXOGEN Ultrasound Bone Healing System is indicated for the non-invasive treatment of established nonunions* excluding skull and vertebra. The EXOGEN device has also been reported as effective as an adjunctive non-invasive treatment of established nonunions in patients:
- With internal or external fracture fixation hardware present. EXOGEN cannot penetrate metal and therefore should not be applied directly over hardware.
- Undergoing treatment for infection at the fracture site. EXOGEN is not intended to treat the infection.
- Believed to have diminished bone quality. EXOGEN is not intended to treat diminished bone quality.
In addition, EXOGEN is indicated for accelerating the time to a healed fracture for fresh, closed, posteriorly displaced distal radius fractures and fresh, closed or Grade I open tibial diaphysis fractures in skeletally mature individuals when these fractures are orthopedically managed by closed reduction and cast immobilization.
There are no known contraindications for the EXOGEN device. Safety and effectiveness have not been established for individuals lacking skeletal maturity, pregnant or nursing women, patients with cardiac pacemakers, on fractures due to bone cancer, or on patients with poor blood circulation or clotting problems. Some patients may be sensitive to the ultrasound gel.
*A nonunion is considered to be established when the fracture site shows no visibly progressive signs of healing.
Full prescribing information can be found in product labelling, at EXOGEN.com, or by calling Customer Service at 1-800-836-4080.
For Providers
Expect More for Your Patients
Many patients have difficult-to-heal fractures, or fractures they want to heal more quickly. EXOGEN offers a non-surgical option that helps to heal nonunion fractures and accelerates indicated acute fractures, so your patients can resume active lifestyles.*1-3
EXOGEN is a low-intensity pulsed ultrasound (LIPUS) bone stimulator with over 25 years of proven noninvasive bone healing.72 Prescribed by more than 10,000 physicians annually to help over a million patients worldwide, EXOGEN has an 86% nonunion heal rate and accelerates the healing of indicated acute fractures by 38%.1-4,12
EXOGEN makes treatment compliance tracking convenient for your patients and verifiable for you, with a built-in treatment tracking calendar.
Explore EXOGEN's Proven Results
The EXOGEN Ultrasound Bone Healing System is the first FDA-approved device with 38% accelerated healing of indicated acute fractures.1,2
Outcomes with EXOGEN:
Accelerated healing in cortical and cancellous bone:
- 58 days and 37 days faster, respectively1,2
- Fracture healing promoted 41% to 51% faster in patients who smoke9
Accelerated healing of indicated fresh tibia fractures in younger and older patients1,52
- 42 days and 84 days faster, respectively1,52
Discover the noninvasive choice for resolution of nonunions. In clinical studies, low-intensity pulsed ultrasound waves helped heal nonunion fractures.
EXOGEN is effective:
- In chronic nonunion fractures that failed to heal at least one year prior to treatment – 86% heal rate35
- In atrophic, hypertrophic, or infected nonunions29,41,53
- With challenging, established nonunions – 86% heal rate3
- With high-energy nonunion fractures – 89% heal rate7
- For use on nonunions with an interfragment gap up to 10 mm and stable osteosynthesis19
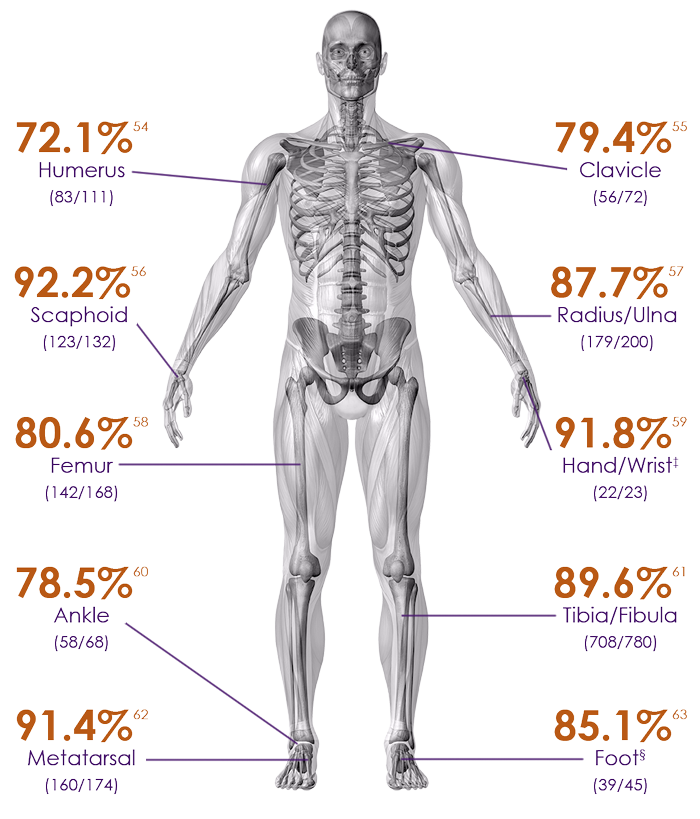
Heal rate of common fractures at risk for nonunions8
In many cases, EXOGEN may prevent the need for further surgery.
Humerus – 72.1% heal rate54
Clavicle – 79.4% heal rate55
Scaphoid – 92.2% heal rate56
Radius/Ulna – 87.7% heal rate57
Femur – 80.6% heal rate58
Hand/wrist – 91.8% heal rate‡59
Ankle – 78.5% heal rate60
Tibia/Fibula – 89.6% heal rate61
Metatarsal – 91.4% heal rate62
Foot – 85.1% heal rate§63
Heal rates based on fractures ages between 91-365 days.54-63
‡Includes metacarpal, carpal and hamate
§Includes talus, calcaneus, tarsal navicular, cuboid and cuneiform
**These studies, which reflect the body of evidence of the bone stimulator EXOGEN, include evaluations of applications outside the approved label. Assignment of evidence levels was based on the updated level of evidence rating system in the Oxford Level of Evidence Criteria. Oxford Centre for Evidence-Based Medicine; www.cebm.ox.ac.uk/resources/levels-of-evidence/ocebm-levels-of-evidence
*Summary of Indications for Use
The EXOGEN Ultrasound Bone Healing System is indicated for the non-invasive treatment of established nonunions† excluding skull and vertebra. The EXOGEN device has also been reported as effective as an adjunctive non-invasive treatment of established nonunions in patients:
- With internal or external fracture fixation hardware present. EXOGEN cannot penetrate metal and therefore should not be applied directly over hardware.
- Undergoing treatment for infection at the fracture site. EXOGEN is not intended to treat the infection.
- Believed to have diminished bone quality. EXOGEN is not intended to treat diminished bone quality.
In addition, EXOGEN is indicated for accelerating the time to a healed fracture for fresh, closed, posteriorly displaced distal radius fractures and fresh, closed or Grade I open tibial diaphysis fractures in skeletally mature individuals when these fractures are orthopedically managed by closed reduction and cast immobilization.
There are no known contraindications for the EXOGEN device. Safety and effectiveness have not been established for individuals lacking skeletal maturity, pregnant or nursing women, patients with cardiac pacemakers, on fractures due to bone cancer, or on patients with poor blood circulation or clotting problems. Some patients may be sensitive to the ultrasound gel.
†A nonunion is considered to be established when the fracture site shows no visibly progressive signs of healing.
Full prescribing information can be found in product labelling, at EXOGEN.com, or by calling Customer Service at 1-800-836-4080.