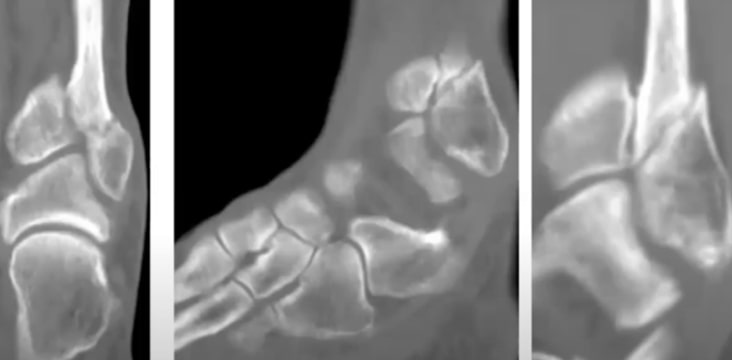
EXOGEN:s funktionsmekanism
Vetenskaplig förklaring av EXOGEN:s unika funktionsmekanism
EXOGEN-prestandaprogrammet Utvärdering
EXOGEN-prestandaprogrammet Registrering
EXOGEN-prestandaprogrammet
Snabbinstruktionsguide
EXOGEN påskyndar läkning av frakturer hos rökare
Dr Zura, ortodped på LSU – LSU Health Sciences Center New Orleans
EXOGEN och frakturläkning i osäkra tider
Dr Zura, ortodped på LSU – LSU Health Sciences Center New Orleans
EXOGEN:s användarguide
Detaljerad information om användning av EXOGEN-systemet
EXOGEN:s tekniker för gipsapplicering
Instruktion för implementering av en givarport i nytt gips eller skapandet av en lucka i befintligt gips
Akuta frakturer
- Low-intensity pulsed ultrasound accelerates rat femoral fracture healing by acting on the various cellular reactions in the fracture callus.
- Acceleration of tibia and distal radius fracture healing in patients who smoke.
- Acceleration of tibial fracture-healing by non-invasive, low-intensity pulsed ultrasound.
- Accelerated healing of distal radial fractures with the use of specific, low-intensity ultrasound. A multicenter, prospective, randomized, double-blind, placebo-controlled study.
- Does low intensity, pulsed ultrasound speed healing of scaphoid fractures?
- The use of low-intensity ultrasound to accelerate the healing of fractures.
Utebliven frakturläkning
- Indications and results for the EXOGEN ultrasound system in the management of nonunion: a 59-case pilot study.
- Low-intensity pulsed ultrasound in the treatment of nonunions.
- Low-intensity pulsed ultrasound: effects on nonunions.
- Improved healing response in delayed unions of the tibia with low-intensity pulsed ultrasound: results of a randomized sham-controlled trial.
- The evaluation of the healing rate of subtalar arthrodeses, part 2: the effect of low-intensity ultrasound stimulation.
- Compound high-energy limb fractures with delayed union: our experience with adjuvant ultrasound stimulation (exogen).
- Failure of surgery for scaphoid nonunion is associated with smoking.
- Treatment of chronic (>1 year) fracture nonunion: heal rate in a cohort of 767 patients treated with low-intensity pulsed ultrasound (LIPUS).
Övrigt
- Mode & mechanism of low intensity pulsed ultrasound (LIPUS) in fracture repair.
- Prolonged endochondral bone healing in senescence is shortened by low-intensity pulsed ultrasound in a manner dependent on COX-2.
- Sources of information influencing decision-making in orthopaedic surgery – an international online survey of 1147 orthopaedic surgeons.
- Design evolution enhances patient compliance for low-intensity pulsed ultrasound device usage.
EXOGEN-prestandaprogrammet är ett program som administrerar återbetalning av kostnaden för EXOGEN till köpare vid utebliven läkning enligt kriterierna nedan. Programmet är även utformat för att uppmuntra och motivera patienterna till att följa den ordinerade behandlingen.
Kriterier
Alla patienter som har köpt en EXOGEN-enhet som utformats för behandling av en fraktur som tidigare ordinerats av en kvalificerad läkare i syfte att behandla en stabil, icke-rubbad, fastställd utebliven frakturläkning,* fördröjd läkning eller akut fraktur med en frakturspricka på mindre än 10 millimeter (exklusive kot- och skallfrakturer) är berättigade. Alla patienter kommer att registreras för programmet automatiskt.
Patienter måste behandla frakturen med EXOGEN enligt produktinstruktionerna i minst 120 dagar i följd och uppnå en ordinationsföljsamhet på minst 90 %.
Utvärdering
För att du ska vara berättigad måste din ordinerande läkare fastställa att ditt ben inte har läkt eller bildat sammanläkning av benvävnad. Ordinerande läkare måste tillhandahålla två röntgenbilder av ditt berörda ben, en bild före användning av EXOGEN och en till bild efter minst 120 dagars behandling med EXOGEN som visar saknad sammanläkning av benvävnad.
EXOGEN-enheten har en intern patientanvändningsmonitor som registrerar datum, tid och varaktighet för varje behandlingssession. Denna monitor kommer användas av Bioventus för att bekräfta att en behandlingsföljsamhet på minst 90 % uppfylls.
Undantag
- Frakturtyper:
- Ostabila frakturer
- Rubbade frakturer
- Frakturer med frakturspricka över 10 millimeter
- Kot- och skallfrakturer
- Patologiska frakturer
- Modifierade eller ändrade enheter
- Patienter som behandlas med modell EXOGEN 4000+
- EXOGEN-prestandaprogrammet blir ogiltigt om alternativa behandlingar genomförs under behandlingsperiodens 120 dagar. Om alternativ behandling behövs påbörjas en ny behandlingsperiod på 120 dagar
- EXOGEN måste köpas och tas emot av patienten direkt från från en auktoriserad distributör av EXOGEN
- EXOGEN-prestandaprogrammet är endast avsett för de patienter som enheten ordinerats till
- Övriga kostnader som är förknippade med inköpet: Endast patientens egen betalning till den auktoriserade distributören kommer att återbetalas
Om du vill verifiera att du är kvalificerad för programmet, besök sidan Kontakta oss för distributörens information. Bioventus hanterar inte dessa anspråk direkt. Du behöver endast kontakta distributören.
Anspråk måste tas emot inom ett år efter det första EXOGEN-behandlingsdatumet.
Bioventus eller distributören förbehåller sig rätten att när som helst ändra eller avbryta programmet.
- EXOGEN:s eftermarknadsapp för data innehåller verklig benläkningsstatistik som du kan använda som riktmärken för dina specifika patienter.
- Det är vår kostnadsfria smartphoneapp som automatiskt visar den läkningsfrekvens som är kopplad till vanliga frakturer, inberäknat patientriskfaktorer och andra relevanta kliniska överväganden.
- Använd EXOGEN:s eftermarknadsapp för data till att hitta nästa lämpliga patient.
- Ladda ned EXOGEN:s eftermarknadsapp för data.
Summering av användningsområde
EXOGEN indiceras för icke-invasiv behandling av bendefekter (förutom ryggrad och skalle), som omfattar behandling av försenad läkning, utebliven frakturläkning,* stressfrakturer och ledfusion. EXOGEN indiceras även för påskyndning av läkningstiden för färska frakturer, reparation efter osteotomi, reparation i bentransportingrepp och reparation i osteodistraktionsingrepp.
Det finns inga kända kontraindikationer för EXOGEN-anordningen. Säkerhet och effektivitet har inte fastställts för individer vars skelett är omoget, gravida eller ammande kvinnor, patienter med hjärtpacemaker, frakturer från bencancer eller patienter med dålig blodcirkulation eller koagulationsproblem. En del patienter kan vara känsliga för ultraljudsgelen.
Fullständig ordinationsinformation finns på produktmärkningen och på EXOGEN.com, Du kan även ringa Bioventus kundtjänst1-800-836-4080.
*En utebliven frakturläkning anses etablerad när frakturstället inte visar några synliga tecken på läkning.